Hepcidin peptidomimetics that are orally stable and systemically active will mark a paradigm change in management of blood disorders that exhibit aberrant iron homeostasis (e.g. hereditary hemochromatosis) and in conditions that can be influenced by modulating stressed iron homeostasis (e.g. polycythemia vera). Hepcidin modulates the iron exporter membrane protein ferroportin and is the master regulator of iron homeostasis in the body. Orally bioavailable "Minihepcidins" have been previously shown to be efficacious in lowering serum iron in mice when dosed peroral (PO) (Preza GC et. al., Journal of Clinical Investigation 2011). Here we describe hepcidin mimetic peptides that are metabolically stable in the gastrointestinal tract, systemically absorbed when delivered orally, and pharmacodynamically active in reducing serum iron parameters in pre-clinical models. Further, we also demonstrate improvement in disease parameters in a mouse model for hereditary hemochromatosis.
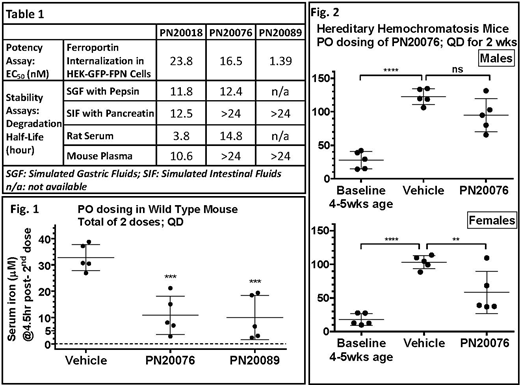
The oral peptides, PN20076 and PN20089, have EC50 of 16.5 nM and 1.39 nM respectively in cell based ferroportin internalization assay (Table 1). In comparison EC50 was 67.8 nM for Hepcidin and 6.12 nM for PTG-300. (PTG-300 is an injectable hepcidin mimetic currently in Phase 2 clinical studies for polycythemia vera and hereditary hemochromatosis.) Oral stability of the peptides was evaluated in a panel of assays, including in vitro matrices simulating the gastric and intestinal conditions, and ex vivo matrices of serum/plasma from different species. Table 1 shows data for peptides PN20018, PN20076 and PN20089. PN20076 demonstrated extended stability in gastric and intestinal conditions, and degradation half-life of >24 hr in mouse plasma and 14.8 hr in rat serum. Based on their stability and potency data from the above battery of screening assays, the peptides were selected for in vivo evaluation in healthy mice to characterize their pharmacodynamic (PD) and pharmacokinetic (PK) properties. PN20076 and PN20089 showed equivalent PD response of reduction in serum iron concentration in wild type mice. After two successive PO doses of PN20076 or PN20089 approximately 24 hr apart, serum iron concentration was reduced from ~30 µM to ~10 µM (group averages), i.e. ~66% reduction, at 4.5 hr post-second dose for both peptides (Fig. 1). At 4.5 hr post-dose, the serum concentration of PN20076 was ~262 nM.
PN20076 was further evaluated for its effect in lowering iron overload in a mouse model for hemochromatosis (HFE2-/- with homozygous deletion of hemojuvelin, a positive regulator of hepcidin expression). This mouse model is marked by hyper-absorption of dietary iron, higher transferrin saturation and deposition of excessive iron in liver, all manifestations of aberrant iron homeostasis caused by the genetic disruptions of the hepcidin-iron pathway. Liver iron accumulation was significantly prevented in groups treated with PN20076 once daily (QD) by PO administration for over two weeks, as compared to vehicle treated controls (Fig. 2). The reduction in non-heme iron concentration in liver homogenates (measured using a colorimetric iron assay) was statistically significant in the female group treated with PN20076.
We have described orally stable and systemically active hepcidin mimetic peptides and demonstrated oral activity in preventing liver iron overload in hemochromatosis mice. The effective reduction of iron absorption from the diet and the steady state lowering of transferrin-saturation can potentially prevent tissue iron toxicity in hereditary hemochromatosis. Similarly, the sustained reduction of systemic iron levels with an oral hepcidin mimetic to control stressed iron homeostasis should reduce excessive erythrocytosis, a hallmark of polycythemia vera and other congenital and acquired erythropoietic disorders.
Bourne:Protagonist Therapeutics: Current Employment, Other: shareholder. Zhang:Protagonist Therapeutics: Current Employment, Other: shareholder. Frederick:Protagonist Therapeutics: Current Employment, Other: shareholder. Tran:Protagonist Therapeutics: Current Employment, Other: shareholder. Vengalam:Protagonist Therapeutics: Current Employment, Current equity holder in private company. McMahon:Protagonist Therapeutics: Current Employment, Other: shareholder. Huie:Protagonist Therapeutics: Current Employment, Other: shareholder. Ledet:Protagonist Therapeutics: Current Employment, Other: shareholder. Zhao:Protagonist Therapeutics: Current Employment, Other: shareholder. Tovera:Protagonist Therapeutics: Current Employment, Current equity holder in private company. Lee:Protagonist Therapeutics: Current Employment, Current equity holder in private company. Yang:Protagonist Therapeutics: Current Employment, Other: shareholder. Dion:Protagonist Therapeutics: Current Employment, Current equity holder in private company. Yuan:Protagonist Therapeutics: Current Employment, Other: shareholder. Zemede:Protagonist Therapeutics: Current Employment, Current equity holder in private company. Nguyen:Protagonist Therapeutics: Current Employment, Current equity holder in private company. Masjedizadeh:Protagonist Therapeutics: Current Employment, Current equity holder in private company. Cheng:Protagonist Therapeutics: Current Employment, Current equity holder in private company. Mattheakis:Protagonist Therapeutics: Current Employment, Current equity holder in private company. Liu:Protagonist Therapeutics: Current Employment, Current equity holder in private company. Smythe:Protagonist Therapeutics: Current Employment, Other: shareholder.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal